
Quite notably, all thermodynamic potentials are expressed in terms of conjugate pairs. In thermodynamics, the internal energy of a system is expressed in terms of pairs of conjugate variables, for instance, pressure/volume or temperature/entropy. The force per unit area applied on a surface in a direction perpendicular to that surface in question.

The measure of the tendency of an object or system to spontaneously give up energy. The quantification of how much space an object occupies. The thermodynamic state of a gas is described by three main characteristics: This amount of heat is defined by a theory known as a Maxwell-Boltzmann Distribution, which expresses the distribution of energy among the molecules of a gas during thermal equilibrium.įor all intents and purposes, the gas phase is a completely disordered state whereby, in accordance with the Second Law of Thermodynamics, gas particles immediately diffuse to homogeneously fill any shape or volume of space made available to them. Gas particles move at a high rate of speed, based upon the substance's temperature. The interactions they do have are rare and random in a collision-like manner. There are no resistive forces either binding them together or pulling them apart.
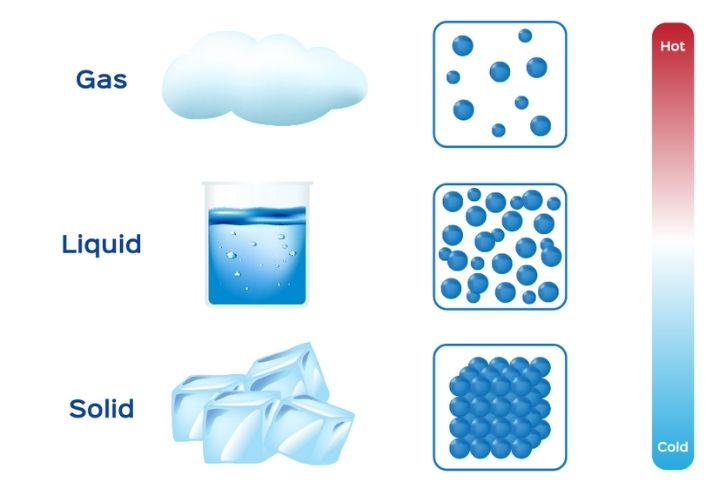
In a gaseous state, the atoms in the substance move in an independent fashion. Should the gas be further heated, its atoms or molecules can become (wholly or partially) ionized, thus, in turn, turning the gas into a plasma.

This shortcut process is known as sublimation. For example, carbon dioxide can go from being a solid to a gas without ever being a liquid. However, some have the ability to transform without going through each stage. Typically, substances will go through the stages in order: solid, liquid, gas. As heat (energy) is added, the solid melts and, typically, first becomes a liquid, and then, eventually, a gas. 5) Plasmas are ionized (electrically charged) gases that possess an equal number of positive and negative charges, examples include fluorescent lighting and the sun.Ī notable fact about gases is that they often tend to appear in solid form until subjected to increased temperature levels.


 0 kommentar(er)
0 kommentar(er)
